The interaction between the Central Nervous System (CNS) and the Immune System is critical to maintaining normal health. A fundamental aspect of this interaction is the presence of the Blood Brain Barrier (BBB), the physical separation between the vascular system and the tissues of the brain and spinal cord. The BBB works to prevent the diffusion of toxins, bacteria, antibodies and large proteins from crossing the barrier while simultaneously allowing the passage of small hydrophobic substances such as O2, CO2 and hormones in cerebrospinal fluid (CSF)[1].
Microglia act as the first line of active immune defense for the CNS. In the brain and spinal cord, microglia work as phagocytes to remove damaged neurons, plaques and infectious agents. Antigenic activation of microglia may also stimulate an increase in astrocyte activity and vice-versa. Together, astrocytes and microglia differentially regulate trafficking of lymphocyte subsets across brain endothelial cells[2].
A second mode of interaction between the CNS and the immune system occurs when an antigen crosses the BBB and infiltrates nervous tissue. In order to defend against such an attack, the CNS has countermeasures in place to halt and eliminate antigens. Effector and memory T cells constantly survey the CNS to protect from infection and the manifestation of cancer[1]. This neuroinflammatory response, if unregulated, may be detrimental to the CNS[1,3].
Upon injury to the CNS, neuroinflammation leads to infiltration of CD4 activated cells survival signals for T cells. During the inflammatory response, TGF-β secreted by astrocytes and glial cells is joined by IL-6 or IL-21 to form T-cells (TH-17). These T cells produce cytokines such as IL-17, IL-17F and IL-22 to regulate the inflammatory response within the CNS[1,3].
A third mode of interaction between the CNS and immune system is the effect of hormones and cytokines on overall immune function. The immune response triggers the Hypothalamic-Pituitary-Adrenal axis (HPA axis), which initiates the release of glucocorticoids such as cortisol. Cortisol limits the cytokine induced inflammatory response and prevents an overreaction from the immune system. This chain of events has been interpreted to indicate that the immune system can act as a sensory system, notifying the brain of the presence of a threat and triggering a classical stress response[4].
Of the large number of known cytokines, the most relevant to the nervous system are IL-1, IL-6, TNFa, and the interferons. IL-1 is a considerably more potent activator of adrenocorticotropic hormone (ACTH) and glucocorticoid secretion than CRF itself[4]. Cytokines within the CNS activate immune responses that elicit tissue repair or cell damage and cytotoxicity resulting in necrosis and loss of oligodendrocytes[1,4].
The interactions between the CNS and immune system are very complex. Signaling by the HPA axis and its effects over the CNS and immune system are involved in regulating interactions between immune cells, their substances, and the CNS. A delicate balance must be maintained between the aforementioned components of the immune system and CNS in order to ensure a quality state of health for the patient.
1. Multiple Sclerosis
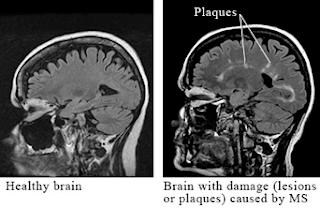
Multiple sclerosis is a chronic inflammatory disease characterized by recurrent episodes of demyelination that disturbs bundles of nerves in the white matter of the central nervous system. Components of the CNS affected by the presence of sclerotic plaques include the cerebellum, spinal cord, and projections of optic nerve, somatosensory pathways, costicospinal tract and basal ganglia [see fig. 2]. The cause of this disease is unknown but it is believed that it could be a T cell-mediated autoimmune disease caused by a combination of factors including heredity, susceptibility to infectious agents, production of autoantibodies by autoreactive B-cells against myelin antigens such as myelin basic protein, myelin oligodendrocyte glycoprotein and proteolipid protein [5]. In addition, increased frequency of developing the disease is associated with individuals that carry the HLA-2 or HLA-3 genes, genes that encode receptors for IL-2 or IL-7 [6], as well as relative lack of vitamin D and exposure to cold and temperate climates [6]. The symptoms are characterized by partial or complete loss of vision, muscle weakness, fatigue, numbness and problems with balance, speech, and generalized motor coordination, including bowel and urinary control [7, 9] [see fig. 1]. In 50% of patients the cumulative sensory and motor losses may lead to generalize muscular paralysis, thus requiring help for walking within 15 years of initial occurrence of symptoms [9]. Even though there is no cure, treatments involving administration of corticosteroids and interferon β-1a or β-1b have shown to reduce the progression of the disease.
Figure 1
(Multiple Sclerosis Symptoms. Health Kut. http://healthkut.com/blog/wp-content/uploads/2010/02/multiple_sclerosis-Symptoms.png. Accessed April 29, 2010.)
Figure 2
(Brain Scan Lesion Damage. Health.com. http://www.health.com/health/static/hw/media/medical/hw/h9991221.jpg. Accessed April 29, 2010.)
2. Myasthenia Gravis
Myasthenia gravis is an autoimmune disease where antibodies produced by T and B-lymphocytes reduces the number of functional nicotinic acetylcholine (Ach) receptors in the postsynaptic membrane of the neuromuscular junction [see fig.4]. This causes a decrease of response of the muscle fibers to Ach, thus eliciting a progressive muscular weakness. It has been established that molecular mimicry plays an important factor in this disease due to structural similarities in amino acid sequences shared with certain poliovirus proteins. Also, individuals carrying HLA-DR3 gene have increase risk of expressing the disease [11]. Clinical features of Myasthenia Gravis involve general muscle weakness that greatly manifests in the muscles of the arms, head and chest [9]. It characterizes by fatigue involving muscles of the eyes and eyelids, causing ptosis [see fig. 3], facial expression and of the pharynx, leading to problems with mastication, swallowing, holding the head upright and in speech [8, 9]. In severe cases of the disease, muscular weakness of the diaphragm and abdominal intercostal muscles produces respiratory paralysis, with a mortality rate of 5-10% [9]. It has been reported that 70% of patients with this disease have an abnormal thymus, therefore thymectomy, as well as administration of corticosteroids and immunosuppressant therapy is recommended [8, 9, 11]. However, an important therapeutic approach involves the administration of anticholinesterase drugs, such as neostigmine, which reduces the activity of cholinesterase, thus allowing the rising of Ach at the synapse in order to stimulate the existing receptors and producing muscle contraction.
Figure 3
(Ptosis (Drooping Eye). ADAM. http://wendyusuallywanders.files.wordpress.com/2008/01/mgdroop.jpg. Accessed April 29, 2010.)
(Myasthenia Gravis. Journal of the American Medical Association. http://jama.ama-assn.org/content/vol298/issue1/images/medium/jmn70071fa.jpg. Accessed April 29, 2010)
3. Lupus
Systemic Lupus Erythematosus (SLE) is an autoimmune disorder that is characterized by a multisystem microvascular inflammation with the generation of autoantibodies. The precise reason for the abnormal autoimmunity that causes lupus is not known. Patients with lupus produce abnormal antibodies in their blood that target tissues within their own body rather than foreign infectious agents. Many immune disturbances, both innate and acquired, occur in SLE, as illustrated in below[10].
(Bartels C, MD. Systemic Lupus Erythematosus. 2009. Medscape http://emedicine.medscape.com/article/332244-overview.)
It has been suggested that the development of autoantibodies involves a defect in apoptotic cells, it has been demonstrated that there is clustering of lupus autoantigens in the surface blebs of apoptotic cells. Thus, intolerant lymphocytes begin targeting normally protected intracellular antigens [10].
Lupus can cause disease of the skin, heart, lungs, kidneys, joints, and/or nervous system. Immune complexes form in the microvasculature, leading to complement activation and inflammation. Moreover, antibody-antigen complexes deposit on the basement membranes of skin and kidneys. In active SLE, this process has been confirmed based on the presence of complexes of nuclear antigens such as DNA, immunoglobulins, and complement proteins at these sites. Serum antinuclear antibodies are found in virtually all individuals with active SLE, and antibodies to native double-stranded DNA are relatively specific for the diagnosis of SLE[10].
Questions
1. What is the role of microglia in Central Nervous System infection?
2. How do T cells protect the CNS if an antigen crosses the blood- brain barrier?
3. What is the role of the endocrine system in CNS infection?
4. Which of the following is true regarding microglial cells:
a) Derived from neural crests
b) Work as phagocytes in the CNS
c) Interact directly with T cells to protect against infection
d) Are found in the peripheral nervous system
5. Which one of the following cells is responsible for protection of the CNS from infections and the manifestation of cancer?
a) Microglial cells
b) Astrocytes
c) Glial cells
d) Effector or memory T-cells
6. Which of the following pertains to Myasthenia Gravis
a) Treatment includes an acetylcholinesterase agonist
b) The Ca2+ channels at the presynaptic membrane of the neuromuscular junction are affected
c) A well-known cure for myasthenia gravis is the surgical removal of the thymus (thymectomy)
d) Autoimmune disease against nicotinic acetylcholine (Ach) receptors in the postsynaptic membrane of the neuromuscular junction
References:
1. Fabry Z, Schreiber HA, Harris MG, Sandor M. Sensing the microenvironment of the central nervous system: Immune cells in the central nervous system and their pharmacological manipulation. Current Opinion in Pharmacology. 2008; 8(4): 496-507. Available at http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2614337/pdf/nihms71472.pdf/?tool=pmcentrez. Accessed April 15, 2010.
2. Graeber MB, Streit WJ. Microglia: biology and pathology. Acta Neuropathologica. 2010; 119: 89-105. Available at http://www.springerlink.com/content/b0026l22p2347125/. Accessed April 15,2010.
3. Schmitz T, Chew L. Cytokines and myelination in the central nervous system. Scientific World Journal. 2008;8:1119-1147. Available at http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2663591/?tool=pmcentrez doi: 10.1100/tsw.2008.140. Accessed April 16, 2010.
4. Dunn AJ. Interactions Between the Nervous System and the Immune System. Psychopharmacology – 4th Generation of Progress. 2000. Available at http://www.acnp.org/G4/GN401000069/CH069.html. Accessed April 15,2010.
5. Murphy, K., Travers, P., & Walport, M. (2008). Janeway's Immunobiology. New York, NY: Garland Science.
6. Multiple Sclerosis (MS) and Related Disorders. The Merck Manuals web site. http://www.merck.com/mmhe/sec06/ch092/ch092b.html. Accessed April 15, 2010.
7. Goverman J. Autoimmune T cell responses in the central nervous system. National Institute of Health. 2009; 9(6). Available at http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2813731/pdf/nihms170109.pdf/?tool=pmcentrez doi: 10.1038/nri2550. Accessed April 15,2010.
8. Neuromuscular Junction Disorders. The Merck Manuals website. http://www.merck.com/mmhe/sec06/ch095/ch095c.html?qt=myasthenia%20gravis&alt=sh#sec06-ch095-ch095c-1391. Accessed April 15, 2010
9. Martini F, Welch K. A&P applications manual. San Francisco, CA: Benjamin Cummings. 2006.
10. Bartels C, MD. Systemic Lupus Erythematosus. 2009. Medscape http://emedicine.medscape.com/article/332244-overview.
11.Doan, T., Melvold, R., Viselli, S., & Waltenbaugh, C. (2008). Lippincott's illustrated reviews: immunology. Philadelphia, PA: Lippincott Williams & Wilkins.




No comments:
Post a Comment